top of page
Expert Chemistry, Biology, Physics and Math Tutoring Online and Bethesda,MD
646-407-9078
Search
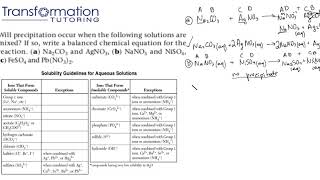

Will precipitation occur when the following solutions are mixed?
Will precipitation occur when the following solutions are mixed? If so, write a balanced chemical equation for the reaction. (a) Na2CO3...
3,039 views0 comments


A sample of the male sex hormone testosterone,C19H28O2, contains 3.88 * 10^21 hydrogen atoms.
A sample of the male sex hormone testosterone,C19H28O2, contains 3.88 * 10^21 hydrogen atoms. (a) How many atoms of carbon does it...
4,962 views0 comments


How To Name Acids
How To Name Acids An acid is a substance that releases H+ ions (hydrogen ions) when it is dissolved in water. Acids can be divided into 2...
41 views0 comments


How to convert moles into mass (grams)?
How to convert moles into mass? To convert moles into mass, we first need to find the molar mass of the compound given. This can be done...
1,905 views0 comments


How do we know if the reaction is spontaneous?
What does it mean for a reaction to be spontaneous? It means that the reaction will occur on its own, without any outside energy input...
88 views0 comments


How to calculate pH of the weak acid.
When we are asked to find pH, the first thing we need to recognize is whether we are dealing with acid or base? Also we need to...
21 views0 comments
State the general trend in first ionization energy as the elements in Period 3 are considered from l
First ionization energy is the amount of energy needed to take first electron from an atom. The trend for ionization energy is that it...
695 views0 comments
Equivalence point on titration curves.
Titration is a laboratory procedure where a known base is added to unknown acid (or vise versa) to figure out the molarity of the...
8 views0 comments
How do intermolecular forces affect vapor pressure?
What is vapor pressure? The simple way to understand it, is to realize that pressure is caused by gas (in our case vapor). The more gas...
1,899 views0 comments
Identify a Brønsted-Lowry conjugate acid-base pair in the reaction.
CH3CH2COOH(aq) + H2O(l) ---> CH3CH2COO−(aq) + H3O+(aq) The key to idenitifying Bronsted-Lowry acid-base pair and realizing that acid and...
1,469 views0 comments
Looking for Chemistry Tutoring?
I tutor all levels of chemistry including general and organic chemistry.
bottom of page
