top of page
Expert Chemistry, Biology, Physics and Math Tutoring Online and Bethesda,MD
646-407-9078
Search


Use VSEPR to predict bond angles about each atom of carbon, nitrogen, and oxygen in these molecules
Use VSEPR to predict bond angles about each atom of carbon, nitrogen, and oxygen in these molecules. Do you need help with general or...
173 views
0 comments


Are the structures in each set valid contributing structures
Are the structures in each set valid contributing structures? Rules for resonance: Only electrons are allowed to move Second Row Elements...
25 views
0 comments
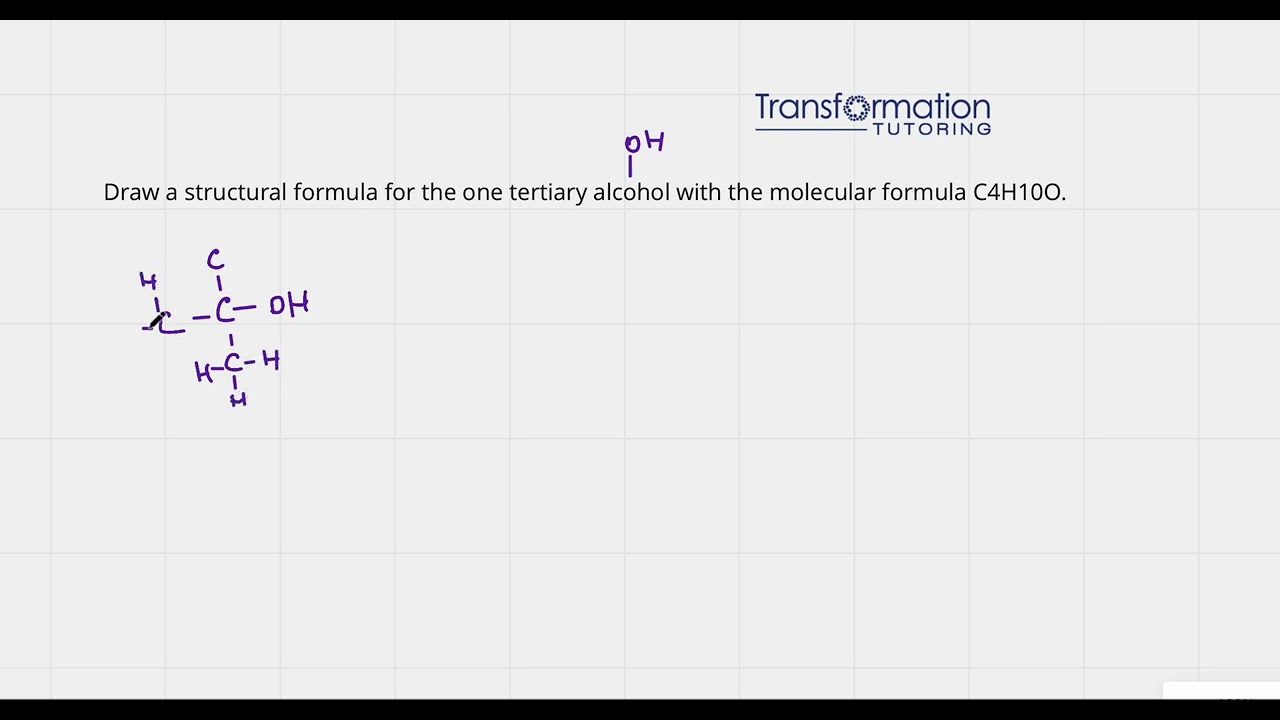

Draw a structural formula for the one tertiary alcohol with the molecular formula C4H10O
An alcohol functional group is OH. A tertiary alcohol is when an OH group is connected to a carbon that is connected to 3 other carbons....
97 views
0 comments
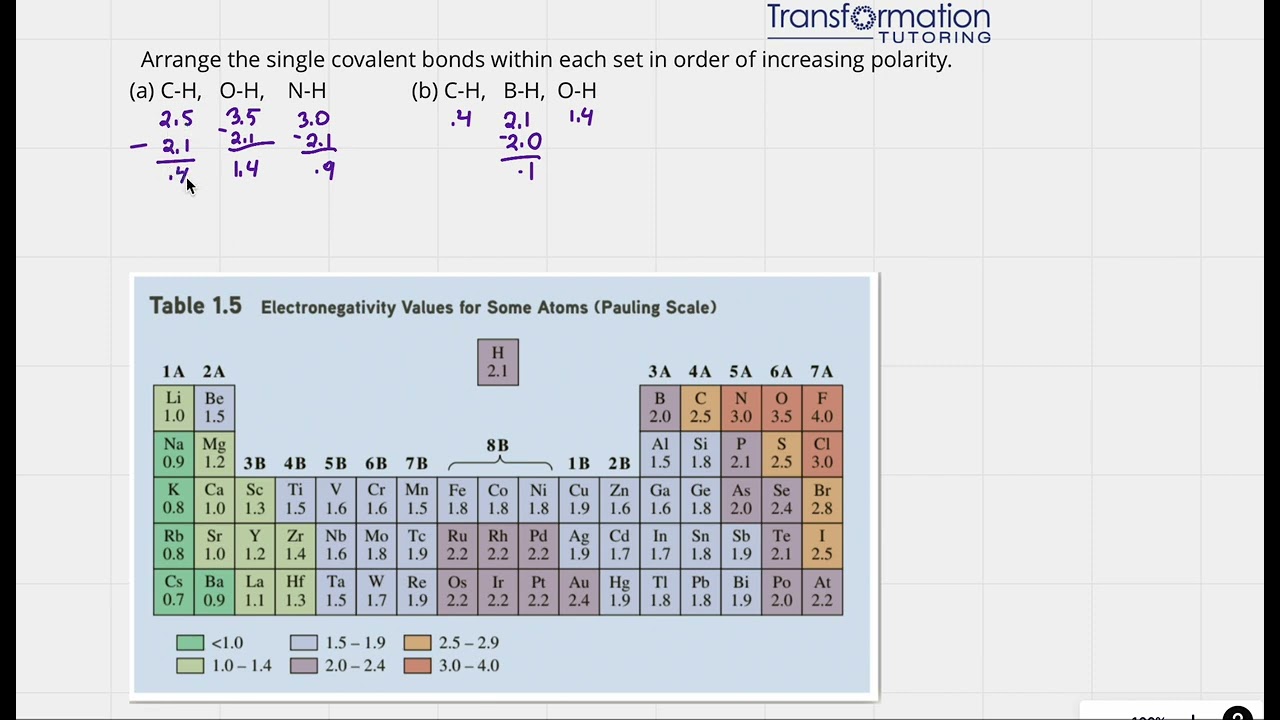

How To Arrange Covalent Bonds In Order Of Increasing Polarity?
Arrange the single covalent bonds within each set in order of increasing polarity. (a) C-H, O-H, N-H (b) C-H, B-H, ...
794 views
0 comments
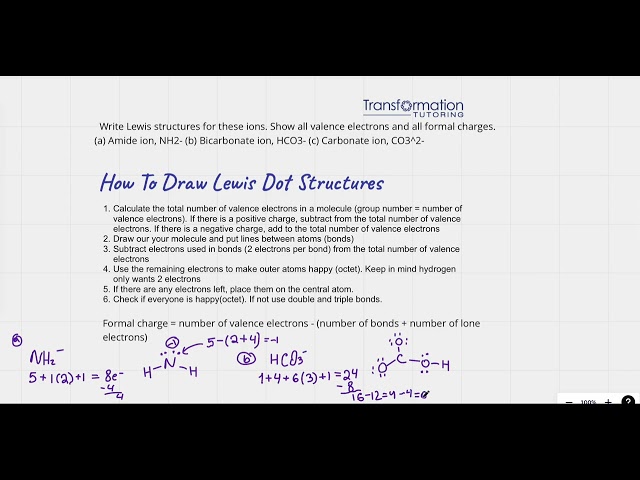

How To Write Lewis Dot Structure For Ions And Show Their Formal Charges?
Write Lewis structures for these ions. Show all valence electrons and all formal charges. (a) Amide ion, NH2- (b) Bicarbonate ion, HCO3-...
66 views
0 comments


How To Write Lewis Dot Structures With Examples H2O2, N2H4, CH3OH
Write Lewis structures for these compounds. Show all valence electrons. None of them contains a ring of atoms. (a) Hydrogen peroxide,...
60 views
0 comments


How To Add Electrons To Complete Octet And Assign Formal Charges (with examples)
Following the rule that each atom of carbon, oxygen, and nitrogen reacts to achieve a complete outer shell of eight valence electrons,...
14 views
0 comments


Indicate the direction of polarity, if any, in each covalent bond
Using the symbols ∂- and ∂+, indicate the direction of polarity, if any, in each covalent bond. a. C-Cl b. S-H c. C-S d. P-H Are...
14 views
0 comments


How many electrons are in the valence shell of each atom?
How many electrons are in the valence shell of each atom? (valence electrons) (a) Carbon (b) Nitrogen (c) Chlorine (d) Aluminum Are you...
4 views
0 comments


Identify the atom that has each ground-state electron configuration
Identify the atom that has each ground-state electron configuration. (a) 1s2 2s2 2p6 3s2 3p4 (b) 1s2 2s2 2p4 In this video we learn 2...
7 views
0 comments
Looking for Chemistry Tutoring?
I tutor all levels of chemistry including general and organic chemistry.
bottom of page
