SYNTHESIS OF EPOXIDES
Peroxyacid Epoxidation
In this reaction an alkene is converted into an epoxide with the use of peroxyacid reagent, RCO3H (peroxyacid). Popular reagents include CF3CO3H and mCPBA
This epoxidation takes place in a one step concerted mechanism. Therefore, the stereochemistry of the double bond substituents remains the same.
Product: Turn double bond into a single bond and attach the two carbons that had a double bond to an oxygen, making an epoxide. The epoxide can be on a wedge (both carbons attached to the oxygen on a wedge) or dashed.

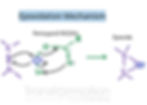
BASE-PROMOTED CYCLIZATION OF HALOHYDRINS
In this reaction, the starting molecule needs to have an OH group and a halogen (Br, Cl, I) on neighboring carbons (halohydrin). Moreover, OH and the halogen need to be trans in order for this reaction to occur (SN2 will occur). The most popular way to make this is to use alkene with Br2, H2O or Cl2, H2O which gives a halohydrin.
Now, lets get to the actual reaction.
First, OH gets deprotonated by a strong base such as OH-. Then, O- attacks the carbon with the halogen from the back via SN2 reaction and the halogen leaves, creating an epoxide.

REACTIONS OF EPOXIDES
Reactions of epoxide can be divided into two categories: acid catalyzed epoxide opening and base catalyzed epoxide opening. It is very important to determine which reagent we are dealing with; acid or base. The product of epoxide opening is an OH and a nucleophile on neighboring carbons with TRANS stereochemistry.
ACID CATALYZED RING OPENING OF EPOXIDES
Under acidic conditions, first epoxide gets protonated by the acid. Then, the nucleophile attacks the MOST substituted carbon from the epoxide and the bond between oxygen and carbon breaks. This is an SN2 reaction that results in the inversion of stereochemistry on the carbon that is being attacked.
The product is an OH group on the least substituted carbon from the epoxide and nucleophile on the most substituted carbon from the epoxide. Stereochemistry is trans.
Examples of common acids include: HCN, HCl, HBr, HI, H3O+, ROH with acid

BASE CATALYZED RING OPENING OF EPOXIDES
Under basic conditions, the base attacks the LEAST substituted carbon on the epoxide. The bond between carbon and oxygen breaks, making O-. Finally, O- gets protonated in the last step to make OH. The product is OH on the most substituted carbon from the epoxide and base attached to the least substituted carbon from the epoxide carbons. Stereochemistry is trans.

REACTIONS OF EPOXIDES WITH GRIGNARD AND ORGANOLITHIUM REAGENTS
Grignard and organolihtium reagents are both strong bases and react the same way at base catalyzed ring opening of epoxides. The final product will be OH on the most substituted carbon from the epoxide and R group on the least substituted carbon from the epoxide. Stereochemistry will be trans.

REACTION OF EPOXIDES WITH LiAlH4 (lithium aluminum hydride)

Reaction of epoxide with LiAlH4 reduces it to an alcohol. LiAlH4 is a donor of H- (hydride ion), which attacks epoxide from the least substituted carbon. The other carbon from the epoxides receives a hydrogen.
Are you looking for an online organic chemistry tutor? Click HERE
We also have: General chemistry tutor online
